Synaptic changes lie at the nexus of many neurodegenerative diseases, underpinning cognitive decline. However, it has been hard to get a handle on exactly how these tiny structures change in disease, because scientists have had no way to examine expression changes in single synapses at scale. In the January 16 Nature Biotechnology, researchers led by David Weitz at Harvard University and Chenghang Zong at Baylor College of Medicine in Houston present a high-throughput way to profile the RNA composition of thousands of individual synapses. Going by the mouthful Multiple-Annealing-and-Tailing-based Quantitative scRNA-Seq in Droplets, or MATQ-Drop, the method isolates nuclei or synaptosomes from brain, encases each in an oil droplet that adds a unique barcode, and then mixes the labeled droplets for one large-scale RNA-Seq.
- New method permits high-throughput RNA-Seq of single synapses.
- It identifies distinct synaptic profiles in mouse and in human hippocampus.
- With amyloidosis, inflammatory and complement transcripts shoot up.
With this approach, the authors identified 12 distinct synaptic profiles in wild-type mouse hippocampus, and six in postmortem human hippocampus, confirming that synapses within a brain region vary at the RNA level. The scientists think they might uncover more human profiles if the postmortem interval were shorter. In the 5XFAD mouse model of amyloidosis, the 12 synaptic subtypes expressed higher levels of inflammatory factors, especially complement, than did synapses from control mice.
“This approach opens a whole new avenue of biology for neuroscientists to look into synapses at the individual level,” Zong told Alzforum.
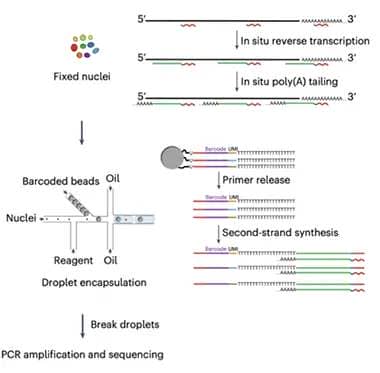
Two-Step RNA-Seq. Used with either isolated nuclei (pictured) or synaptosomes, MATQ-Drop starts with internal primers to reverse-transcribe mature, immature, and noncoding RNA; in the second step, beads add a unique barcode to individual nuclei or synaptosomes in separate oil droplets. [Courtesy of Niu et al., Science Advances/AAAS.]
Others agreed. “This technology may help us better understand the diversity of synapses in the brain in health, and how they change in disease,” Robert Vassar at Northwestern University, Chicago, wrote to Alzforum. Ulrich Hengst at Columbia University, New York, noted differences in transcript splicing among synapses. “The paper provides several intriguing starting points for future research,” Hengst said (full comments below).
Why analyze RNA in synapses? Previous work has found that neurons shuttle transcripts to both pre- and post-synapses where regulated local translation allows for rapid change in protein composition in response to stimuli (May 2019 news). Those studies examined synapses in bulk, however, making it impossible to discern whether there were subtypes that might have specific agendas. To achieve single-synapse resolution, the authors combined two existing methods, the MATQ-Seq technology developed by Zong, and the droplet sequencing method developed by Weitz and colleagues at Harvard (Sheng et al., 2017; Sheng and Zong, 2019; Macosko et al., 2015).
MATQ uses primers that bind at several sites along a transcript, rather than only at the poly-A tail. This allows for reverse transcription of total RNA, including immature, unspliced, and long noncoding RNAs (lncRNA), as well as mature transcripts. LncRNAs serve as scaffolds for transcripts and help regulate gene expression. Applying the MATQ technique to synapses was challenging, however, because these structures are open rather than membrane-bound like a nucleus, which means RNA leaks out. To prevent this, first author Muchun Niu fixed synaptosomes immediately after isolating them, locking RNA in place before reverse transcription. Then the authors used fluorescence-activated sorting to separate synaptosomes, merging each one with a drop of oil containing bar-coded oligonucleotides. Enzymes in the oil added the oligos to the cDNA strands from the reverse transcription step, labeling each synapse’s transcriptome before large-scale sequencing.
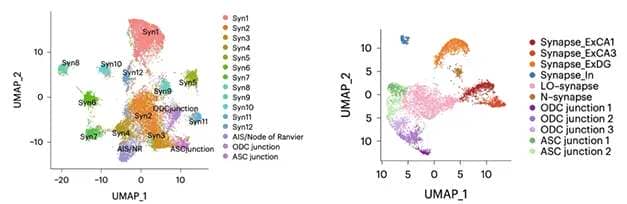
Mouse versus Human. In mouse hippocampus, MATQ-Drop found 12 distinct synaptic signatures (left), plus neuron-oligodendrocyte (ODC), neuron-astrocyte (ASC), and axon initial segment (AIS) signatures. In human hippocampus, six synaptic, three ODC, and two ASC junction signatures were captured (right). [Courtesy of Niu et al., Science Advances/AAAS.]
Applying this method to mouse hippocampus, the authors identified 12 distinct synaptic profiles, or “synaptomes.” They also found axon initial segments, as well as what the authors called neuron-astrocyte and neuron-oligodendrocyte “junctions,” i.e., places where these cell types meet. The junctions appeared because the technique for isolating synaptosomes picked up other subcellular structures as well.
Two of the synaptic profiles contained transcripts for known presynaptic markers such as PCLO and BSN, and one contained postsynaptic markers, such as SHANK1, but the nature of the other subtypes was less clear. Notably, each synaptic subtype had a distinct signature of unspliced mRNA and of lncRNA as well. Comparing these synaptic profiles to single-nuclei RNA-Seq from the same hippocampal samples, the authors found that while there was some correspondence between synaptic and neuronal subtype transcriptomes, they did not match up cleanly. This implies that some synaptic subtypes are common to several types of neuron.
In postmortem hippocampus and prefrontal cortex samples from two human brains, the researchers found fewer RNA profiles, but these matched up better with neuron transcriptome subtypes. Three synaptomes came from excitatory neurons, two from inhibitory, and one seemed to mark immature synapses. The differences between mouse and human samples might be partly due to the long postmortem interval for the human samples, allowing RNA to decay, the authors speculated. As in mouse, they detected neuron-astrocyte and neuron-oligodendrocyte “junctions,” as well as subtype-specific lncRNAs.
What happens in AD? In 20,000 hippocampal synaptosomes isolated from two 5XFAD and two wild-type mice, transcripts for inflammatory genes, particularly complement proteins, shot up. C1q quadrupled compared to wild-type synapses. Complement proteins, especially C1q, have been implicated in synapse loss in AD (Nov 2015 conference news; Jun 2022 news). The authors found other changes as well, such as more myelination transcripts, and a switch in the type of calcium sensor from Camk2d to Camk2a, potentially affecting synaptic plasticity (Zalcman et al., 2018). Crucially, these synaptic changes were not reflected in single-nuclei RNA-Seq data from the same samples, indicating that synaptic profiling captures unique data.
Finally, the authors compared MATQ-Drop to a commercial platform for single-nuclei RNA-Seq, the 10x Genomics Chromium. When both methods were applied to nuclei isolated from mouse hippocampus, MATQ-Drop was more sensitive, detecting a third more unique neuronal transcripts and more than twice as many glial transcripts as did 10x Genomics Chromium. “Because of our total RNA capture, we have much more efficient gene detection compared to the latest commercial platforms,” Niu said.—Madolyn Bowman Rogers
Research Models Citations
- 5xFAD (C57BL6)
News Citations
- Plasticity Hums With Protein Synthesis on Both Sides of Synapse
- Microglia Control Synapse Number in Multiple Disease States
- C1q Shows Promise as Therapeutic Target to Stop Synapse Loss
Paper Citations
-
Sheng K, Cao W, Niu Y, Deng Q, Zong C.
Effective detection of variation in single-cell transcriptomes using MATQ-seq.
Nat Methods. 2017 Mar;14(3):267-270. Epub 2017 Jan 16
PubMed. -
Sheng K, Zong C.
Single-Cell RNA-Seq by Multiple Annealing and Tailing-Based Quantitative Single-Cell RNA-Seq (MATQ-Seq).
Methods Mol Biol. 2019;1979:57-71.
PubMed. -
Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, Trombetta JJ, Weitz DA, Sanes JR, Shalek AK, Regev A, McCarroll SA.
Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets.
Cell. 2015 May 21;161(5):1202-1214.
PubMed. -
Zalcman G, Federman N, Romano A.
CaMKII Isoforms in Learning and Memory: Localization and Function.
Front Mol Neurosci. 2018;11:445. Epub 2018 Dec 4
PubMed.



